The Biosimilars Initiative was launched in 2019 to expand health care services in B.C. by switching patients from biologic (originator) drugs to biosimilar versions shown to be as safe and effective.
 The *Biosimilars Initiative introduces “Biosimilar” medication in place of “Originator” medication (the originator medication must be covered by PharmaCare). These drugs are approved by Health Canada and once introduced there is a transition period of 6 months for a person to move from the originator drug to the biosimilar one. You must ask the prescriber to write you a new prescription for the biosimilar drug prior to the end of the 6 month trial period. After the switch period ends, PharmaCare only covers the biosimilar medication.
The *Biosimilars Initiative introduces “Biosimilar” medication in place of “Originator” medication (the originator medication must be covered by PharmaCare). These drugs are approved by Health Canada and once introduced there is a transition period of 6 months for a person to move from the originator drug to the biosimilar one. You must ask the prescriber to write you a new prescription for the biosimilar drug prior to the end of the 6 month trial period. After the switch period ends, PharmaCare only covers the biosimilar medication.
BC PharmaCare is adding Humira to this list in order to move patients from the more expensive originator version. After the trial period ends, patients will be required to switch to the biosimilar of Humira by October 7, 2021.
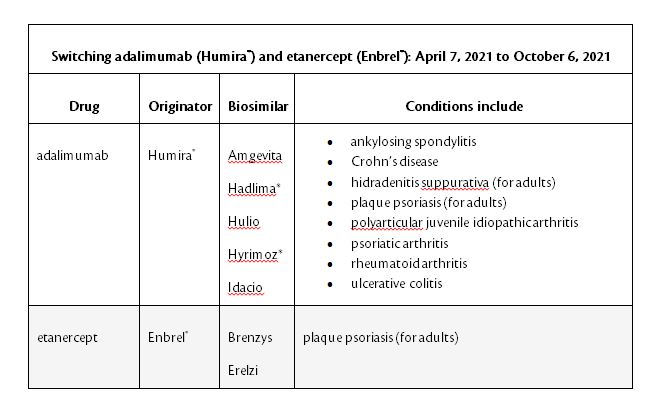
*Hadlima and Hyrimoz are not yet approved to treat pediatric Crohn’s disease.
One carrier in particular; Equitable Life has advised that they will no longer cover Humira for plan members in BC effective October 7, 2021 and will instead cover one of the biosimilars listed above.
*For more information see the Biosimilars FAQ for patients or contact your ENCOMPASS Advisor.


